ocrevus start up form
And sign and date the form or it could delay our ability to help you. Ad Discover The Safety Efficacy Of TYSABRI.

Fillable Online Infusion Checklist For Ocrevus In Relapsing Or Fax Email Print Pdffiller
Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary.

. OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access. Every 6 months infuse 600mg in 500mL of 09 NS. RMS and PPMS and their open-label extensions up to.
View full PI and Boxed Warning. Ad Visit here to discover how AUBAGIO can help. Once we have both.
These infusion reactions can happen for up to 24 hours after your infusion. Send it via fax. A representative from OCREVUS Access Solutions or your.
Prior Authorization Form for. Use this form to learn about your health insurance coverage and financial assistance options and enroll in optional services from Genentech Access Solutions. If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy.
See Website For Safety Boxed Warning PI. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with. According to immunization guidelines live or live-attenuated vaccines should be administered at least 4 weeks prior to initiation of.
It is important that. Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV. The documents accompanying this transmission may contain confidential health information.
Once youve prescribed OCREVUS enroll your patients in OCREVUS Access Solutions. View full PI and Boxed Warning. Ad Get patients started with AUBAGIO.
Instructions for Patients Please write legibly and complete all required fields on the OCREVUS Start Form to prevent delays. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300. View full prescribing information and Boxed Warning.
Sample infusion referral form Please confirm compliance. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin receiving the services it. Genentech can start helping you when page 4 of this form is submitted by you or your doctors office in one of the following ways.
These infusion reactions can happen for up to 24 hours after your infusion. Prescription Enrollment Form. Access the OCREVUS Start Form and learn more about the assistance Genentech offers for your OCREVUS ocrelizumab patients.
Ad Visit here to discover how AUBAGIO can help. Is this a new start or continuation of therapy. View full prescribing information and Boxed Warning.
Swelling of the throat. Access Solutions is committed to helping your patients access the Genentech medicines they need providing assistance to your patients after OCREVUS is prescribed. Inform patients that infusion reactions can occur up to 24.
See Website For Safety Boxed Warning PI. Discover The Answers You Need Here. To a final concentration of 12mgmL.
Discover The Answers You Need Here. OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access. Sign a printed form and fax or mail it to us or give it to your doctors office to do so Your doctor also has to fill out a form called the OCREVUS Start Form.
OCREVUS is a prescription medicine used to treat. Ocrevus ocrelizumab Fax completed form to 8086506487. Ad Get patients started with AUBAGIO.
Ad Discover The Safety Efficacy Of TYSABRI.

Rebif Interferon Beta 1a Relapsing Multiple Sclerosis Rms Treatment

Can You Prevent Multiple Sclerosis Everyday Health
Roche S Ocrevus Achieves Blockbuster Status Pharmaceutical Technology
Progressive Ms Research Multiple Sclerosis Ms International Federation

Ocrevus Side Effects Explained By Neurologist Youtube

Ocrevus Ocrelizumab Access Solutions

Hla Dr15 Molecules Jointly Shape An Autoreactive T Cell Repertoire In Multiple Sclerosis Sciencedirect
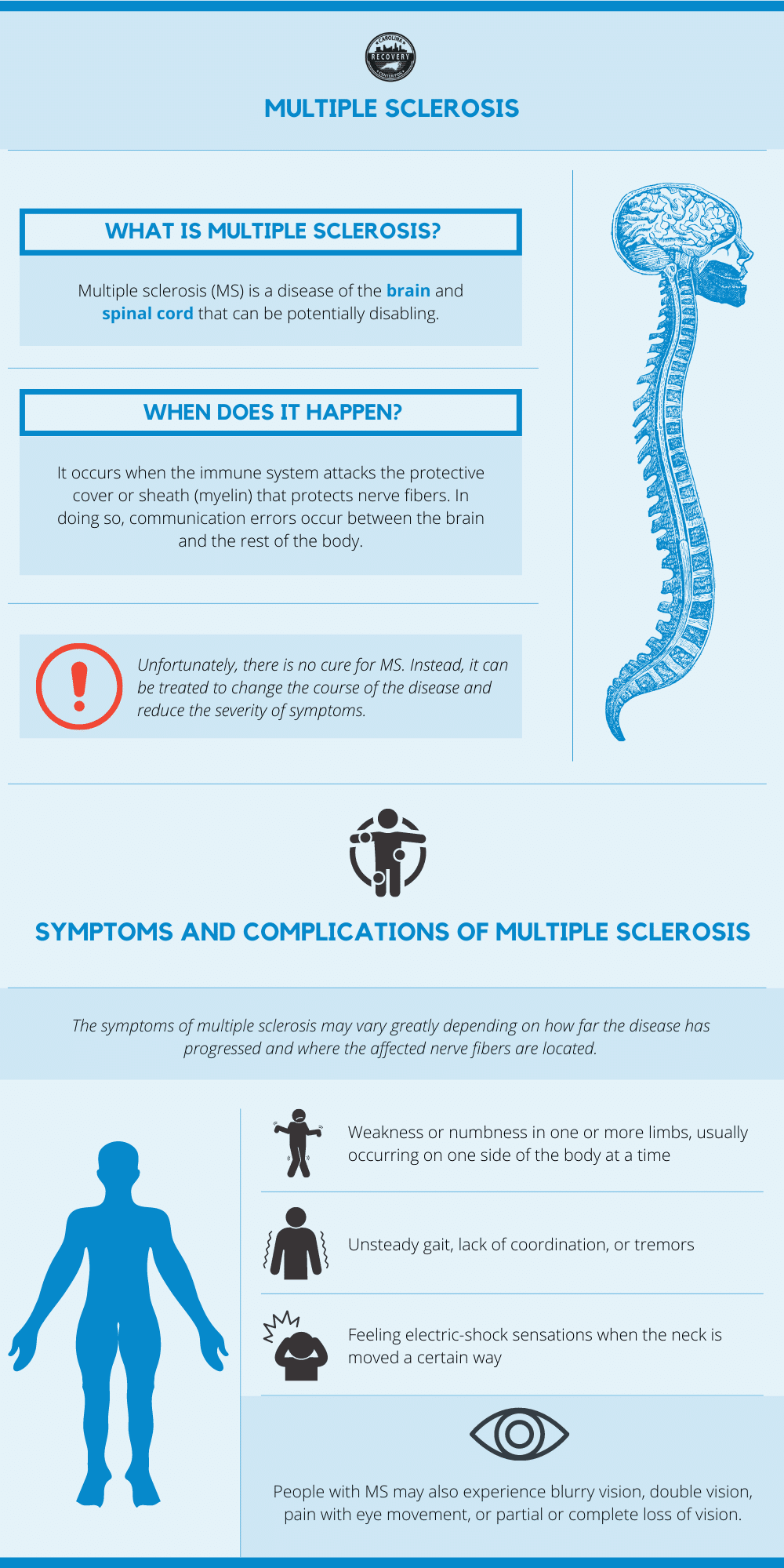
Multiple Sclerosis Ms And Substance Abuse What S The Connection

Ocrevus Ocrelizumab Multiple Sclerosis Ms Treatment
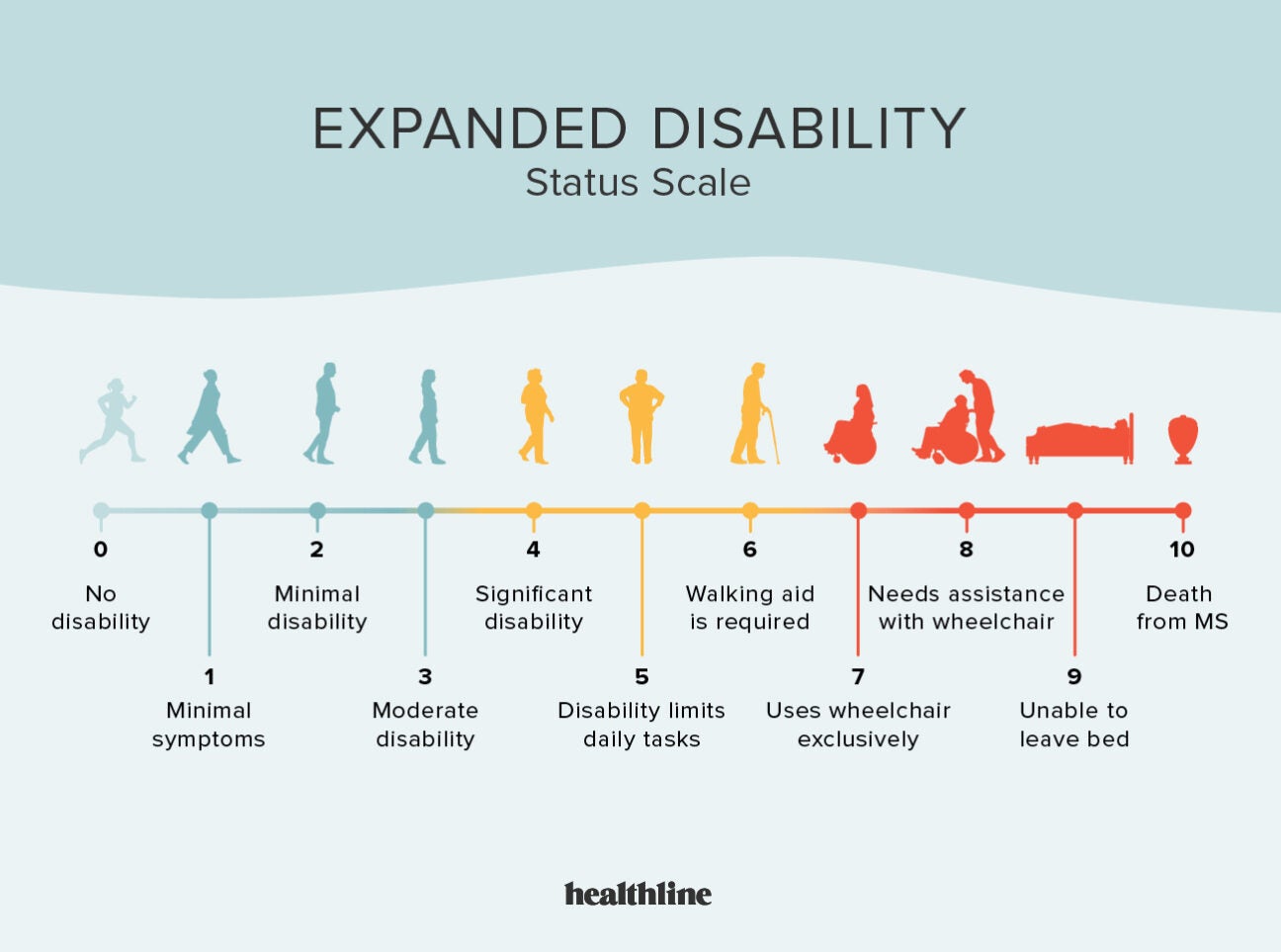
Ms Progression Chart Stages Of Ms Disability Scale And More
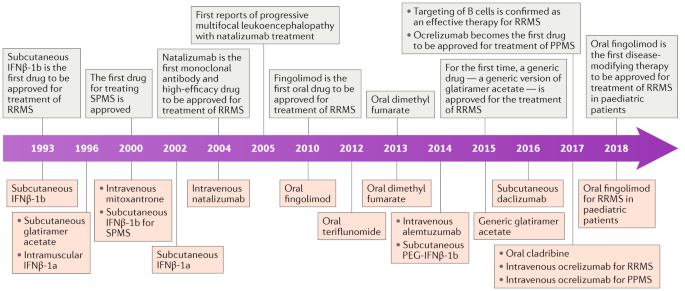
Treatment Of Multiple Sclerosis Success From Bench To Bedside Nature Reviews Neurology

Multiple Sclerosis Where Do Efforts Stand Against Its Elusive Progressive Form Consult Qd
Allergy Drug Improves Function In Patients With Chronic Injury From Multiple Sclerosis Uc San Francisco

Percutaneous Venous Angioplasty In Patients With Multiple Sclerosis And Chronic Cerebrospinal Venous Insufficiency A Randomized Wait List Control Study Annals Of Vascular Surgery
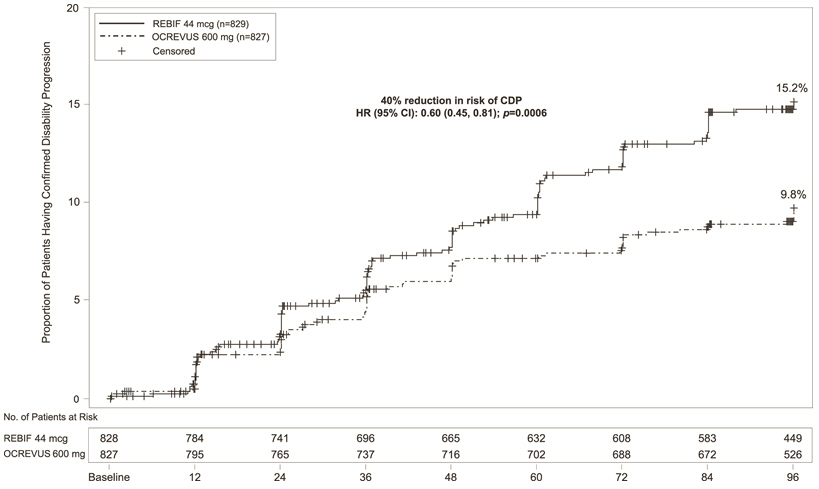
Ocrevus Package Insert Prescribing Information Drugs Com

Ocrevus Ocrelizumab Results For Rms Relapsing Ms

Patients And Caregivers Home Ocrevus Co Pay Program

Timing Of High Efficacy Therapy For Multiple Sclerosis A Retrospective Observational Cohort Study The Lancet Neurology
